Passerini reaction
| Passerini reaction | |
|---|---|
| Named after | Mario Passerini |
| Reaction type | Carbon-carbon bond forming reaction |
| Identifiers | |
| Organic Chemistry Portal | passerini-reaction |
| RSC ontology ID | RXNO:0000244 |
The Passerini reaction is a chemical reaction involving an isocyanide, an aldehyde (or ketone), and a carboxylic acid to form a α-acyloxy amide.[1][2][3][4][5] This addition reaction is one of the oldest isocyanide-based multicomponent reactions and was first described in 1921 by Mario Passerini in Florence, Italy.[6][7] It is typically carried out in aprotic solvents but can also be performed in ionic liquids such as water or deep eutectic solvents.[7] It is a third order reaction; first order in each of the reactants. The Passerini reaction is often used in combinatorial and medicinal chemistry with recent utility in green chemistry and polymer chemistry.[6][8][9] As isocyanides exhibit high functional group tolerance, chemoselectivity, regioselectivity, and stereoselectivity, the Passerini reaction has a wide range of synthetic applications.[6][10][11][12]
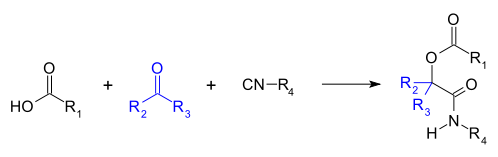
Mechanism
[edit]The Passerini reaction has been hypothesized to occur through two mechanistic pathways.[10][7][11] The reaction pathways are dependent on the solvent used.
Concerted mechanism
[edit]A concerted mechanism, seen in SN2 and Diels−Alder reactions, is theorized to occur when the Passerini reagents are present at high concentration in aprotic solvents.[10]

This mechanism involves a trimolecular reaction between the isocyanide, carboxylic acid, and carbonyl in a sequence of nucleophilic additions. The reaction proceeds first through an imidate intermediate and then undergoes Mumm rearrangement to afford the Passerini product.[13][14]
As the Mumm rearrangement requires a second carboxylic acid molecule, this mechanism classifies the Passerini reaction as an organocatalytic reaction.[14][15]
Ionic mechanism
[edit]
In polar solvents, such as methanol or water, the carbonyl is protonated before nucleophilic addition of the isocyanide, affording a nitrilium ion intermediate. This is followed by the addition of a carboxylate, acyl group transfer and proton transfer respectively to give the desired Passerini product.[11][7]
Reaction control
[edit]Molecular weights of polymers synthesized through the Passerini can be controlled through stoichiometric means.[16] For example, polymer chain length and weight can adjusted through isocyanide stoichiometry, and polymer geometry can be influenced through starting reagents.[16][17] To facilitate the Passerini reaction between bulky, sterically hindered reagents, a vortex fluidic device can be used to induce high shear conditions. These conditions emulate the effects of high temperature and pressure, allowing the Passerini reaction to proceed fairly quickly.[18] The Passerini reaction can also exhibit enantioselectivity. Addition of tert-butyl isocyanide to a wide variety of aldehydes (aromatic, heteroaromatic, olefinic, acetylenic, aliphatic) is achieved using a catalytic system of tetrachloride and a chiral bisphosphoramide which provides good yield and good enantioselectivities.[19] For other types of isocyanides, rate of addition of isocyanide to reaction mixture dictates good yields and high selectivities.[19]
Applications
[edit]Apart from forming α-acyloxy amide products, the Passerini reaction can be used to form heterocycles, polymers, amino acids, and medicinal products.
Heterocycles
[edit]
The original Passerini reaction produces acyclic depsipeptides which are labile in physiological conditions. To increase product stability for medicinal use, post-Passerini cyclization reactions have been used to afford heterocycles such as β-lactams, butenolides, and isocoumarins.[16] To enable these cyclizations, reagents are pre-functionalized with reactive groups (ex. halogens, azides, etc.) and used in tandem with other reactions (ex. Passerini-Knoevenagel, Passerini-Dieckmann) to afford heterocyclic products.[16] Compounds like three membered oxirane and aziridine derivatives, four-membered b-lactams, and five-membered tetrasubstituted 4,5-dihydropyrazoles have been produced through this reaction.[12]
Polymers
[edit]
This reaction has also been used for polymerization, monomer formation, and post-polymerization modification.[20][21][22][17][23] The Passerini reaction has also been used to form sequence-defined polymers.[24] Bifunctional substrates can be used to undergo post-polymerization modification or serve as precursors for polymerization.[10][11][8] As this reaction has high functional group tolerance, the polymers created using this reaction are widely diverse with tuneable properties.[20] Macromolecules that have been produced with this reaction include macroamides, macrocyclic depsipeptides, three-component dendrimers and three-armed star branched mesogen core molecules.[12]
Amino acids and pharmaceuticals
[edit]Passerini reaction has been employed for the formation of structures like α-amino acids, α-hydroxy-β-amino acids, α-ketoamides, β-ketoamides, α-hydroxyketones and α-aminoxyamides.[12] The Passerini reaction has synthesized α-Acyloxy carboxamides that have demonstrated activity as anti-cancer medications along with functionalized [C60]-fullerenes used in medicinal and plant chemistry.[12][25] This reaction has also been used as a synthetic step in the total synthesis of commercially available pharmaceuticals such as telaprevir (VX-950), an antiviral sold by Vertex Pharmaceuticals and Johnson & Johnson.[12]

See also
[edit]References
[edit]- ^ Passerini, M.; Simone, L. Gazz. Chim. Ital. 1921, 51, 126–29.
- ^ Passerini, M.; Ragni, G. Gazz. Chim. Ital. 1931, 61, 964–69.
- ^ Banfi, L.; Riva, R. (2005). The Passerini Reaction. Vol. 65. pp. 1–140. doi:10.1002/0471264180.or065.01. ISBN 978-0471264187.
{{cite book}}:|journal=ignored (help). - ^ Kazemizadeh, A.R.; Ramazani, A. (2012). "Synthetic applications of Passerini reaction". Curr. Org. Chem. 16 (4): 418–450. doi:10.2174/138527212799499868.
- ^ Banfi, L.; Basso, A.; Lambruschini, C.; Moni, L.; Riva, R. (2021). "The 100 facets of the Passerini reaction". Chem. Sci. 12 (47): 15445–15472. doi:10.1039/D1SC03810A. PMC 8654045. PMID 35003575.
- ^ a b c Tuten, Bryan T.; Bui, Aaron H.; Wiedbrauk, Sandra; Truong, Vinh X.; Raston, Colin L.; Barner-Kowollik, Christopher (19 August 2021). "Four component Passerini polymerization of bulky monomers under high shear flow". Chemical Communications. 57 (67): 8328–8331. doi:10.1039/D1CC02984C. ISSN 1364-548X. PMID 34323263. S2CID 236498755.
- ^ a b c d Antenucci, Achille; Marra, Francesco; Dughera, Stefano (2021). "Silica gel-immobilised chiral 1, 2-benzenedisulfonimide: a Brønsted acid heterogeneous catalyst for enantioselective multicomponent Passerini reaction". RSC Advances. 11 (42): 26083–26092. Bibcode:2021RSCAd..1126083A. doi:10.1039/D1RA05297G. PMC 9037113. PMID 35479468.
- ^ a b Abbasi, Elham; Aval, Sedigheh Fekri; Akbarzadeh, Abolfazl; Milani, Morteza; Nasrabadi, Hamid Tayefi; Joo, Sang Woo; Hanifehpour, Younes; Nejati-Koshki, Kazem; Pashaei-Asl, Roghiyeh (21 May 2014). "Dendrimers: synthesis, applications, and properties". Nanoscale Research Letters. 9 (1): 247. Bibcode:2014NRL.....9..247A. doi:10.1186/1556-276X-9-247. ISSN 1556-276X. PMC 4074873. PMID 24994950.
- ^ Dömling, A.; Ugi, I. Angew. Chem. Int. Ed. Engl. 2000, 39, 3168–3210. (Review)
- ^ a b c d The Passirini Reaction L. Banfi, R.Riva in Organic Reactions vol. 65 L.E. Overman Ed. Wiley 2005 ISBN 0-471-68260-8
- ^ a b c d Taran, Jafar; Ramazani, Ali; Joo, Sang Woo; Ślepokura, Katarzyna; Lis, Tadeusz (2014). "Synthesis of Novel a-(Acyloxy)-a-(quinolin-4-yl)acetamides by a ThreeComponent Reaction between an Isocyanide, Quinoline-4-carbaldehyde, and Arenecarboxylic Acids". Helvetica Chimica Acta. 97: 1088–1096. doi:10.1002/hlca.201300378.
- ^ a b c d e f Wahby, Yasmin; Abdel-Hamid, Hamida; Ayoup, Mohammed Salah (2022). "Two decades of recent advances of Passerini reactions: synthetic and potential pharmaceutical applications". New Journal of Chemistry. 46 (4): 1445–1468. doi:10.1039/D1NJ03832J. ISSN 1144-0546. S2CID 245431805.
- ^ Li, Jie Jack (2021), Li, Jie Jack (ed.), "Passerini Reaction", Name Reactions: A Collection of Detailed Mechanisms and Synthetic Applications, Cham: Springer International Publishing, pp. 424–426, doi:10.1007/978-3-030-50865-4_115, ISBN 978-3-030-50865-4, retrieved 24 October 2022
- ^ a b Ramozzi, Romain; Morokuma, Keiji (5 June 2015). "Revisiting the Passerini Reaction Mechanism: Existence of the Nitrilium, Organocatalysis of Its Formation, and Solvent Effect". The Journal of Organic Chemistry. 80 (11): 5652–5657. doi:10.1021/acs.joc.5b00594. ISSN 0022-3263. PMID 25974627.
- ^ Marcelli, Tommaso; Olimpieri, Francesca; Volonterio, Alessandro (29 June 2011). "Domino synthesis of 1,3,5-trisubstituted hydantoins: a DFT study". Organic & Biomolecular Chemistry. 9 (14): 5156–5161. doi:10.1039/C1OB05242J. ISSN 1477-0539. PMID 21643563.
- ^ a b c d Oelmann, S.; Solleder, S. C.; Meier, M. a. R. (1 March 2016). "Controlling molecular weight and polymer architecture during the Passerini three component step-growth polymerization". Polymer Chemistry. 7 (10): 1857–1860. doi:10.1039/C5PY02030A. ISSN 1759-9962.
- ^ a b Rudick, Jonathan G. (October 2013). "Innovative macromolecular syntheses via isocyanide multicomponent reactions". Journal of Polymer Science Part A: Polymer Chemistry. 51 (19): 3985–3991. Bibcode:2013JPoSA..51.3985R. doi:10.1002/pola.26808.
- ^ Tuten, Bryan T.; Bui, Aaron H.; Wiedbrauk, Sandra; Truong, Vinh X.; Raston, Colin L.; Barner-Kowollik, Christopher (2021). "Four component Passerini polymerization of bulky monomers under high shear flow". Chemical Communications. 57 (67): 8328–8331. doi:10.1039/D1CC02984C. PMID 34323263.
- ^ a b Denmark, Scott E.; Fan, Yu (1 November 2005). "Catalytic, Enantioselective α-Additions of Isocyanides: Lewis Base Catalyzed Passerini-Type Reactions". The Journal of Organic Chemistry. 70 (24): 9667–9676. doi:10.1021/jo050549m. ISSN 0022-3263. PMID 16292793.
- ^ a b Kreye, Oliver; Tóth, Tommy; Meier, Michael A. R. (16 February 2011). "Introducing Multicomponent Reactions to Polymer Science: Passerini Reactions of Renewable Monomers". Journal of the American Chemical Society. 133 (6): 1790–1792. doi:10.1021/ja1113003. ISSN 0002-7863. PMID 21265532.
- ^ Li, Lei; Lv, An; Deng, Xin-Xing; Du, Fu-Sheng; Li, Zi-Chen (28 August 2013). "Facile synthesis of photo-cleavable polymers via Passerini reaction". Chemical Communications. 49 (76): 8549–8551. doi:10.1039/C3CC44557G. ISSN 1364-548X. PMID 23945608.
- ^ Sehlinger, Ansgar; Kreye, Oliver; Meier, Michael A. R. (13 August 2013). "Tunable Polymers Obtained from Passerini Multicomponent Reaction Derived Acrylate Monomers". Macromolecules. 46 (15): 6031–6037. Bibcode:2013MaMol..46.6031S. doi:10.1021/ma401125j. ISSN 0024-9297.
- ^ Travanut, Alessandra; Monteiro, Patrícia F.; Oelmann, Stefan; Howdle, Steven M.; Grabowska, Anna M.; Clarke, Philip A.; Ritchie, Alison A.; Meier, Michael A. R.; Alexander, Cameron (March 2021). "Synthesis of Passerini-3CR Polymers and Assembly into Cytocompatible Polymersomes". Macromolecular Rapid Communications. 42 (6): 2000321. doi:10.1002/marc.202000321. ISSN 1022-1336. PMID 33249682. S2CID 225447799.
- ^ Solleder, Susanne C.; Meier, Michael A. R. (13 January 2014). "Sequence Control in Polymer Chemistry through the Passerini Three-Component Reaction". Angewandte Chemie International Edition. 53 (3): 711–714. doi:10.1002/anie.201308960. PMID 24307280.
- ^ Ravanello, Bruno B.; Seixas, Nalin; Rodrigues, Oscar E. D.; da Silva, Rafael S.; Villetti, Marcos A.; Frolov, Andrej; Rivera, Daniel G.; Westermann, Bernhard (11 July 2018). "Diversity Driven Decoration and Ligation of Fullerene by Ugi and Passerini Multicomponent Reactions". Chemistry – A European Journal. 24 (39): 9788–9793. doi:10.1002/chem.201802414. PMID 29882608. S2CID 46969435.
