Xylazine
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Rompun, Anased, Sedazine, Chanazine, others |
| AHFS/Drugs.com | International Drug Names |
| License data | |
| Routes of administration | By mouth, inhalation, or injection (intravenous, intramuscular, or subcutaneous) |
| ATCvet code | |
| Legal status | |
| Legal status | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.028.093 |
| Chemical and physical data | |
| Formula | C12H16N2S |
| Molar mass | 220.33 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Xylazine is a structural analog of clonidine and an α2-adrenergic receptor agonist,[1] sold under many trade names worldwide, most notably the Bayer brand name Rompun,[2] as well as Anased, Sedazine and Chanazine.[3]
Xylazine is a common veterinary drug used for sedation, anesthesia, muscle relaxation, and analgesia in animals such as horses, cattle, and other mammals.[2] In veterinary anesthesia, it is often used in combination with ketamine. Veterinarians also use xylazine as an emetic, especially in cats.[4] Drug interactions vary with different animals.[5][6][7]
Xylazine has become a commonly abused street drug in the United States where it is known by the street name "tranq", particularly in the territory of Puerto Rico.[8] The drug is being diverted from stocks for equine veterinarians as well as trafficked in bulk from China to be used as a cutting agent for heroin and fentanyl, causing necrotic skin wounds leading to serious infections and limb amputations[9] as well as other health issues.[10][11][12] Fentanyl mixed with xylazine is known by the street names "sleep-cut", "zombie drug", "Iso" and "tranq dope".[8][13][14][15]
History
[edit]Xylazine was discovered as an antihypertensive agent in 1962 by Farbenfabriken Bayer in Leverkusen, Germany.[1] Accounts of the actions and uses of xylazine in animals were reported as early as the late 1960s and early 1970s.[1] Results from early human clinical studies confirmed that xylazine has several central nervous system depressant effects.[1] Xylazine administration is used for sedation, anesthesia, muscle relaxation, and analgesia.[2] It causes a significant reduction in blood pressure and heart rate in healthy volunteers.[failed verification][16] Xylazine was also studied for use in human beings, but due to hazardous side effects, including hypotension and bradycardia, it was not approved by the Food and Drug Administration (FDA) for human use.[12]
In the United States, xylazine was approved by the FDA only for veterinary use as a sedative, analgesic, and muscle relaxant in dogs, cats, horses, elk, fallow deer, mule deer, sika deer, and white-tailed deer.[1][3] The sedative and analgesic effects of xylazine are related to central nervous system depression. Xylazine's muscle relaxant effect inhibits the transmission of neural impulses in the central nervous system.[17]
In scientific research using animal experiments, xylazine is a component of the most common anesthetic, ketamine-xylazine , to anesthetize rats, mice, hamsters, and guinea pigs.[18]
Veterinary use
[edit]
Xylazine is widely used in veterinary medicine as a sedative, muscle relaxant, and analgesic. It is frequently used in the treatment of tetanus.[1] It is not used in human medical treatment. Xylazine is similar to drugs such as phenothiazines, tricyclic antidepressants, and clonidine.[3] As an anesthetic, it is typically used in conjunction with ketamine.[16] In animals, xylazine may be administered intramuscularly or intravenously.[3] As a veterinary anesthetic, xylazine is typically only administered once for intended effect before or during surgical procedures.[1] α2-Adrenergic receptor antagonists such as atipamezole and yohimbine may be used to reverse the effects of xylazine in animals.[19][20][21]
Side-effects
[edit]Side effects in animals include transient hypertension, hypotension, and respiratory depression.[3] Further, the decrease of tissue sensitivity to insulin leads to xylazine-induced hyperglycemia and a reduction of tissue glucose uptake and utilization.[16] The effects in animals last up to 4 hours.[3]
Pharmacokinetics
[edit]In dogs, sheep, horses, and cattle, the half-life is very short: only 1.21– 5.97 minutes. Complete elimination of the drug can take up to 23 minutes in sheep and up to 49 minutes in horses.[1][3] In young rats the half life is one hour.[18] Xylazine has a large volume of distribution of Vd = 1.9 –2.5 for horses, cattle, sheep, and dogs.[3] Though the peak plasma concentrations are reached in 12 –14 minutes in all species, the bioavailability varies between species.[3] The half life depends on the age of the animal, as age is related to prolonged duration of anesthesia and recovery time.[18] Toxicity occurs with repeated administration, given that the metabolic clearance of the drug is usually calculated as 7– 9 times the half-life, which is 4 to 5 days for the clearance of xylazine.[18]
Pharmacology
[edit]Pharmacodynamics
[edit]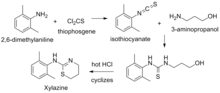
Xylazine is a potent α2-adrenergic receptor agonist.[23][3] When xylazine and other α2-adrenergic receptor agonists are administered, they distribute throughout the body within 30 to 40 minutes.[17] Due to xylazine's highly lipophilic nature, it directly stimulates central α2-adrenergic receptors as well as peripheral α-adrenergic receptors in a variety of tissues.[1][3] As an agonist, xylazine reduces release of norepinephrine and dopamine in the central nervous system.[3] It does so by mimicking norepinephrine in binding to pre-synaptic surface autoreceptors, which leads to feedback inhibition of norepinephrine release.[24]
Xylazine also serves as a transport inhibitor by suppressing norepinephrine transport function through competitive inhibition of substrate transport. Accordingly, xylazine significantly increases Km and does not affect Vmax.[24] This likely occurs by direct interaction on an area that overlaps with the antidepressant binding site.[24] For example, xylazine and clonidine suppress uptake of iobenguane (MIBG), a norepinephrine analogue, in neuroblastoma cells.[24] Xylazine's chemical structure closely resembles clonidine.
It has also been reported that xylazine activates the κ-opioid receptors, with low potency, which may contribute to its effects.[25]
Pharmacokinetics in humans
[edit]Xylazine is absorbed, metabolized, and eliminated rapidly. Xylazine can be inhaled or administered intravenously, intramuscularly, subcutaneously, or orally either by itself or in conjunction with other anesthetics, such as ketamine, barbiturates, chloral hydrate, and halothane in order to provide reliable anesthesia effects.[12][16] The most common route of administration is injection.[12]
Xylazine's action can be seen usually 15–30 minutes after administration and the sedative effect may continue for 1–2 hours and last up to 4 hours.[3] Once xylazine gains access to the vascular system, it is distributed within the blood, allowing xylazine to enter the heart, lungs, liver, and kidney.[26] In non-fatal cases, the blood plasma concentrations range from 0.03 to 4.6 mg/L.[3] Xylazine diffuses extensively and penetrates the blood–brain barrier, as might be expected due to the uncharged, lipophilic nature of the compound.[3]
Xylazine is metabolized by liver cytochrome P450 enzymes.[18] When it reaches the liver, xylazine is metabolized and proceeds to the kidneys to be excreted in urine.[27] Around 70% of a dose is excreted by urine.[18] Thus, urine can be used in detecting xylazine administration because it contains many metabolites, which are the main targets and products in urine.[1][28] Within a few hours, xylazine decreases to undetectable levels.[3] Other factors can also significantly impact the pharmacokinetics of xylazine, such as sex, nutrition, environmental conditions, and prior diseases.[18]
Xylazine Metabolites [28] Xylazine-M (2,6-dimethylaniline) Xylazine-M (N-thiourea-2,6-dimethylaniline) Xylazine-M (sulfone-HO-) isomer 2 Xylazine-M (HO-2,6-dimethylaniline isomer 1) Xylazine-M (HO-2,6-dimethylaniline isomer 2) Xylazine M (oxo-) Xylazine-M (HO-) isomer 1 Xylazine-M (HO-) isomer 1 glucuronide Xylazine-M (HO-) isomer 2 Xylazine-M (HO-) isomer 2 glucuronide Xylazine-M (HO-oxo-) isomer Xylazine-M (HO-oxo-) isomer 1 glucuronide Xylazine-M (HO-oxo-) isomer 2 Xylazine-M (HO-oxo-) isomer 2 glucuronide Xylazine-M (sulfone) Xylazine-M (sulfone-HO-) isomer 1
Recreational use
[edit]In 1979, the first case of xylazine toxicity was reported in a 34-year-old male who had self-medicated for insomnia with injection of 1g of xylazine.[29]
Xylazine is not regulated as a controlled substance under the Controlled Substances Act. It is sold online through distributors often without requiring proof of a veterinary license. As a commonly used veterinary medicine xylazine is probably diverted from veterinary sources. The cost to purchase Xylazine from overseas suppliers is around $6–20 per kilogram. This low price makes it attractive for dealers looking for a cheap additive that is addictive and not treatable with opiate withdrawal medications.[30][31] The withdrawal can last for two weeks and has a quicker onset than fentanyl.[32]
Xylazine is most commonly ingested as an additive with fentanyl.[33] Xylazine has also been reported in combination with medetomidine, another potent α2-adrenergic receptor agonist.[34] It's unknown if drug users are ingesting it knowingly. As of 2024 Seattle police report that some users wrongly believe they are consuming higher quality fentanyl.[35][36][28] Xylazine's street name in Puerto Rico is anestesia de caballo, which translates to "horse anesthetic".[3][37] From 2002 to 2008, its use was associated with a high number of inmate deaths at the Guerrero Correctional Institution in Aguadilla, Puerto Rico.[38]
Xylazine's street name in the United States, particularly when it is mixed with fentanyl, is "tranq", "tranq dope" and "zombie drug".[39]
As of 2012, xylazine users in Puerto Rico were more likely to be male, under age 30, living in a rural area, and injecting rather than inhaling xylazine. Because xylazine and heroin trigger similar behavioral outcomes, the former is often secretly mixed into illegal doses of heroin.[citation needed] The combination of heroin and xylazine produces a potentially more deadly high than administration of heroin alone. Xylazine is also frequently found in "speedball", a mixture of a stimulant drug such as cocaine with a depressant drug such as heroin, morphine and/or fentanyl.[12] As of 2012, causal factors underlying xylazine's increasing popularity were still unknown.[37]
As of 2022, more information on the distribution of xylazine in the body, physical symptoms, and factors predictive of chronic use was known: when used, frequency of use depended on social or economic factors, as well as each user's subjective response to the drug's addictive properties.[40] From November 2021 until August 2022, 80% of drug paraphernalia which tested positive for fentanyl at needle exchange programs in Maryland also contained xylazine.[41] As of 2022, xylazine was almost invariably combined with opioids when used recreationally, and the drug produced a characteristic withdrawal syndrome which complicates treatment of addicted users.[42][43]
In April 2023, the Biden administration declared xylazine-laced fentanyl an official emerging drug threat to the nation, the first time such label has been given. Rahul Gupta, director of the Office of National Drug Control Policy (ONDCP), said he was troubled about what he learned "about the devastating impact of the fentanyl-xylazine combination, which is growing in youth across the nation".[44] According to Gupta, xylazine is the deadliest drug threat the United States has ever faced. The Drug Enforcement Administration (DEA) has seized xylazine and fentanyl mixtures in most states, finding 23% of seized fentanyl powder and 7% of fentanyl pills adulterated with xylazine.[8]
In July 2023, the first death following xylazine use outside of North America was reported to have taken place in Solihull, England in May 22. A 43-year-old male was found dead at home with postmortem toxicology detecting heroin, cocaine, fentanyl and xylazine.[45] Xylazine is anticipated to make inroads in the European illicit drug market once the most recent Afghanistan opium poppy harvest has been produced and delivered, the Taliban having banned poppy cultivation in 2023.[46]
In April 2024, xylazine was reported to be present in illicit THC e-cigarettes in the UK.[47][48]
In April 2024, Seattle police reported that "tranq" was being sold as a standalone narcotic, something they had not seen before. According to Seattle police officials, their patrol officers are now on alert for people collapsing due to tranq consumption.[35] As of May 2024, over 90% of illegally purchased opiates were adulterated with Xylazine in Philadelphia. In Massachusetts, the percentage of samples containing xylazine increased to 42%.[32]
Police departments in 45 US states are preparing for wound care, overdose response, and creating educational materials for communities. There are still surveillance blind spots and, according to one police officer conducting educational outreach with first responders, drug-addicted high school students "[know] more about xylazine than paramedics, nurses and police officers."[32]
Side effects
[edit]Xylazine overdose is often fatal in humans.[1] Because it is used as a drug adulterant, the symptoms caused by the drugs accompanying xylazine administration vary between individuals.[12]
The most common side-effects in humans associated with xylazine administration include bradycardia, respiratory depression, hypotension, transient hypertension secondary to α1-adrenergic receptor stimulation, and other central and hemodynamic changes.[1][12][49] Xylazine significantly decreases heart rate in animals that are not pre-medicated with medications that have anticholinergic effects.[1]
Xylazine administration can lead to diabetes mellitus and hyperglycemia.[16] Other possible side-effects are areflexia, asthenia, ataxia, blurred vision, disorientation, dizziness, drowsiness, dysarthria, dysmetria, fainting, hyporeflexia, slurred speech, somnolence, staggering, coma, apnea, shallow breathing, sleepiness, premature ventricular contraction, tachycardia, miosis and dry mouth.[3] Rarely, hypotonia, urinary incontinence, and nonspecific electrocardiographic ST segment changes occur.[3] Following a human overdose, symptoms can last for 8–72 hours, varying based on xylazine's combined usage with other drugs.[1][3]
Chronic intravenous use of xylazine in combination with opioids is reported to be associated with muscle atrophy, physical deterioration, dependence, abscesses, and skin ulceration, sometimes progressing to necrosis with eschar formation, which can be physically debilitating and painful.[3][16][50] Hypertension followed by hypotension, bradycardia, and respiratory depression lower tissue oxygenation in the skin.[12] Thus, chronic use of xylazine can progress the skin oxygenation deficit, leading to severe skin ulceration.[12] Lower skin oxygenation is associated with impaired healing of wounds and a higher chance of infection.[12] The ulcers may ooze pus and have a characteristic odor.[37] In severe cases, surgical amputations must be performed on the affected extremities.[37]
Overdose
[edit]Since xylazine is not an opioid, it cannot be neutralized by naloxone (Narcan). However, experts still recommend administering naloxone during a suspected xylazine overdose because the drug is very frequently mixed with opioids like fentanyl.[8]
Human tolerance to xylazine varies widely, with toxicity and fatality occurring between doses of 40–2,400 mg (0.62–37.04 gr).[3] Non-fatal blood or plasma concentration ranges from 0.03 to 4.6 mg/L.[26] In fatalities, the blood concentration of xylazine ranges from trace to 16 mg/L.[26] It is reported that there is no defined safe or fatal concentration of xylazine because of the significant overlap between the non-fatal and postmortem blood concentrations of xylazine.[3]
As of 2014, there is no specific antidote to treat humans who overdose on xylazine. Hemodialysis has been suggested as a form of treatment, but is usually unfavorable due to the large volume of distribution of xylazine.[3]
There are no standardized screenings to determine if an overdose has occurred. Detection of xylazine in humans involves various screening methods, such as urine screenings, thin layer chromatography (TLC), gas chromatography–mass spectrometry (GC-MS) and liquid chromatography–mass spectrometry (LC-MS).[28][26] As of November 2022, detecting xylazine in a drug sample requires spectrophotometry.[51]
As of 1998, the α2-adrenergic receptor antagonist atipamezole was used to reverse the effects of xylazine or the related drug dexmedetomidine in veterinary medicine,[52] but this is not an approved medical treatment for humans, despite Phase I clinical trials in 2005.[53]
As of 2001, the effects of xylazine in animals were also reversed by the analeptics 4-aminopyridine, doxapram, and caffeine, which are physiological antagonists to central nervous system depressants.[54] Further research is needed to accurately identify chronic xylazine usage and standardize effective treatments.[1] As of 2014, multiple drugs have been used for therapeutic intervention, including lidocaine, naloxone, thiamine, lorazepam, vecuronium, etomidate, propofol, tolazoline, yohimbine, atropine, orciprenaline, metoclopramide, ranitidine, metoprolol, enoxaparin, flucloxacillin, insulin, and irrigation of both eyes with saline.[3]
The treatment after a xylazine overdose primarily involve maintaining respiratory function and blood pressure.[3] In cases of intoxication, physicians recommend intravenous fluid infusion, atropine, and hospital observation.[1] Severe cases may require tracheal intubation, mechanical ventilation, gastric lavage, activated charcoal, bladder catheterization, electrocardiographic (ECG) and hyperglycemia monitoring.[3] Physicians typically recommend which detoxification treatment should be used to manage possible dysfunction involving highly perfused organs such as the liver and kidneys.[26]
In 2022, the Food and Drug Administration (FDA) issued an alert to American health care providers on the risks patients face if exposed to xylazine in illicit drugs.[55]
References
[edit]- ^ a b c d e f g h i j k l m n o p Greene SA, Thurmon JC (December 1988). "Xylazine—a review of its pharmacology and use in veterinary medicine". Journal of Veterinary Pharmacology and Therapeutics. 11 (4): 295–313. doi:10.1111/j.1365-2885.1988.tb00189.x. PMID 3062194.
- ^ a b c "Xylazine". drugs.com.
- ^ a b c d e f g h i j k l m n o p q r s t u v w x y z aa Ruiz-Colón K, Chavez-Arias C, Díaz-Alcalá JE, Martínez MA (July 2014). "Xylazine intoxication in humans and its importance as an emerging adulterant in abused drugs: A comprehensive review of the literature". Forensic Science International. 240: 1–8. doi:10.1016/j.forsciint.2014.03.015. PMID 24769343.
- ^ Dowling PM (June 2016) [March 2015]. "Drugs to control or stimulate vomiting (monogastric)". Merck Veterinary Manual (professional ed.). Rahway, NJ: Merck & Co.
- ^ Haskins SC, Patz JD, Farver TB (March 1986). "Xylazine and xylazine-ketamine in dogs". American Journal of Veterinary Research. 47 (3): 636–641. PMID 3963565.
- ^ Muir WW, Skarda RT, Milne DW (February 1977). "Evaluation of xylazine and ketamine hydrochloride for anesthesia in horses". American Journal of Veterinary Research. 38 (2): 195–201. PMID 842917.
- ^ Aithal HP, Pratap AK, Singh GR (1997). "Clinical effects of epidurally administered ketamine and xylazine in goats". Small Ruminant Research. 24 (1): 55–64. doi:10.1016/s0921-4488(96)00919-4.
- ^ a b c d "DEA Reports Widespread Threat of Fentanyl Mixed with Xylazine". DEA. 2023-03-21. Archived from the original on 2023-03-21. Retrieved 2023-04-12.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ^ "Recommendations for Caring for Individuals with Xylazine-Associated Wounds". Department of Public Health - City of Philadelphia. Retrieved 11 August 2024.
- ^ Quynh J (October 4, 2023). "Feds target Chinese based fentanyl supply chain with ties to Florida". CBS News. Miami. Retrieved May 24, 2024.
- ^ Rowland B (October 3, 2023). "Justice Department goes after China-based companies in fentanyl fight". The Center Square. Retrieved May 24, 2024.
- ^ a b c d e f g h i j Reyes JC, Negrón JL, Colón HM, Padilla AM, Millán MY, Matos TD, et al. (June 2012). "The emerging of xylazine as a new drug of abuse and its health consequences among drug users in Puerto Rico". Journal of Urban Health. 89 (3): 519–526. doi:10.1007/s11524-011-9662-6. PMC 3368046. PMID 22391983.
- ^ "FDA warns about the risk of xylazine exposure in humans, November 8, 2022". fda.gov. Archived from the original on 2023-03-21. Retrieved 2023-04-12.
Reports from social media and news outlets suggest that xylazine-containing products may be sold under the street names tranq, tranq dope, sleep-cut, Philly dope and zombie drug.
- ^ "FDA alerts health care professionals of risks to patients exposed to xylazine in illicit drugs". www.fda.gov. 2023-03-21. Archived from the original on 2023-03-21. Retrieved 2023-04-12.
- ^ Montero F, Bourgois P, Friedman J (2022). "Potency-Enhancing Synthetics in the Drug Overdose Epidemic: Xylazine ("Tranq"), Fentanyl, Methamphetamine, and the Displacement of Heroin in Philadelphia and Tijuana". Journal of Illicit Economies and Development. 4 (2): 204–222. doi:10.31389/jied.122. PMC 10065983. PMID 37009634.
- ^ a b c d e f Xiao YF, Wang B, Wang X, Du F, Benzinou M, Wang YX (October 2013). "Xylazine-induced reduction of tissue sensitivity to insulin leads to acute hyperglycemia in diabetic and normoglycemic monkeys". BMC Anesthesiology. 13 (1): 33. doi:10.1186/1471-2253-13-33. PMC 4016475. PMID 24138083.
- ^ a b Delehant TM, Denhart JW, Lloyd WE, Powell JD (2003). "Pharmacokinetics of xylazine, 2,6-dimethylaniline, and tolazoline in tissues from yearling cattle and milk from mature dairy cows after sedation with xylazine hydrochloride and reversal with tolazoline hydrochloride". Veterinary Therapeutics. 4 (2): 128–134. PMID 14506588.
- ^ a b c d e f g Veilleux-Lemieux D, Castel A, Carrier D, Beaudry F, Vachon P (September 2013). "Pharmacokinetics of ketamine and xylazine in young and old Sprague-Dawley rats". Journal of the American Association for Laboratory Animal Science. 52 (5): 567–570. PMC 3784662. PMID 24041212.
- ^ Kollias-Baker CA, Court MH, Williams LL (September 1993). "Influence of yohimbine and tolazoline on the cardiovascular, respiratory, and sedative effects of xylazine in the horse". Journal of Veterinary Pharmacology and Therapeutics. 16 (3): 350–358. doi:10.1111/j.1365-2885.1993.tb00182.x. PMID 8230406.
- ^ Murahata Y, Miki Y, Hikasa Y (October 2014). "Antagonistic effects of atipamezole, yohimbine, and prazosin on xylazine-induced diuresis in clinically normal cats". Canadian Journal of Veterinary Research. 78 (4): 304–315. PMC 4170770. PMID 25356000.
- ^ Janssen CF, Maiello P, Wright MJ, Kracinovsky KB, Newsome JT (March 2017). "Comparison of Atipamezole with Yohimbine for Antagonism of Xylazine in Mice Anesthetized with Ketamine and Xylazine". Journal of the American Association for Laboratory Animal Science. 56 (2): 142–147. PMC 5361038. PMID 28315642.
- ^ US expired 4614798A, Elliott, Richard L. & Ruehle, Paul H., "Process for the production of xylazine", issued 30 September 1986, assigned to Vetamix (expired 9 April 2005).
- ^ Hsu WH (July 1981). "Xylazine-induced depression and its antagonism by alpha adrenergic blocking agents". The Journal of Pharmacology and Experimental Therapeutics. 218 (1): 188–192. PMID 6113279.
- ^ a b c d Park JW, Chung HW, Lee EJ, Jung KH, Paik JY, Lee KH (April 2013). "α2-Adrenergic agonists including xylazine and dexmedetomidine inhibit norepinephrine transporter function in SK-N-SH cells". Neuroscience Letters. 541: 184–189. doi:10.1016/j.neulet.2013.02.022. PMID 23485735. S2CID 46338840.
- ^ Bedard ML, Huang XP, Murray JG, Nowlan AC, Conley SY, Mott SE, et al. (2024-05-03). "Xylazine is an agonist at kappa opioid receptors and exhibits sex-specific responses to opioid antagonism". Addiction Neuroscience. 11: 100155. doi:10.1016/j.addicn.2024.100155. PMC 11290297.
- ^ a b c d e Silva-Torres L, Veléz C, Alvarez L, Zayas B (2014). "Xylazine as a drug of abuse and its effects on the generation of reactive species and DNA damage on human umbilical vein endothelial cells". Journal of Toxicology. 2014: 492609. doi:10.1155/2014/492609. PMC 4243599. PMID 25435874.
- ^ Barroso M, Gallardo E, Margalho C, Devesa N, Pimentel J, Vieira DN (April 2007). "Solid-phase extraction and gas chromatographic–mass spectrometric determination of the veterinary drug xylazine in human blood". Journal of Analytical Toxicology. 31 (3): 165–169. doi:10.1093/jat/31.3.165. PMID 17579964.
- ^ a b c d Meyer GM, Maurer HH (December 2013). "Qualitative metabolism assessment and toxicological detection of xylazine, a veterinary tranquilizer and drug of abuse, in rat and human urine using GC-MS, LC-MSn, and LC-HR-MSn". Analytical and Bioanalytical Chemistry. 405 (30): 9779–9789. doi:10.1007/s00216-013-7419-7. PMID 24141317. S2CID 23164588.
- ^ Carruthers SG, Nelson M, Wexler HR, Stiller CR (October 1979). "Xylazine hydrochloridine (Rompun) overdose in man". Clinical Toxicology. 15 (3): 281–285. doi:10.3109/15563657908989878. PMID 509891.
- ^ "Xylazine in Minnesota" (PDF). Minnesota Department of Health.
- ^ "The Growing Threat of Xylazine and its Mixture with Illicit Drugs" (PDF). Drug Enforcement Administration. USDOJ.
- ^ a b c "What is 'tranq' and why do police carry wound care kits because of it?". Masslive. May 9, 2024. Retrieved 11 August 2024.
- ^ Hoffman GR, Giduturi C, Cordaro NJ, Yoshida CT, Schoffstall AM, Stabio ME, et al. (May 15, 2024). "Classics in Chemical Neuroscience: Xylazine". ACS Chem Neurosci. 15 (11): 2091–2098. doi:10.1021/acschemneuro.4c00172.
- ^ de Andrade Horn P, Berida TI, Parr LC, Bouchard JL, Jayakodiarachchi N, Schultz DC, et al. (October 2024). "Classics in Chemical Neuroscience: Medetomidine". ACS Chem Neurosci. doi:10.1021/acschemneuro.4c00583.
- ^ a b Rose D (April 7, 2024). "Seattle Police warn that base ingredient in 'zombie drug' tranq is being sold as standalone pill". Fox 13 Seattle.
- ^ "Xylazine". Office of Addiction Services and Supports (OASAS) - New York. OASAS. Retrieved 11 August 2024.
- ^ a b c d Torruella RA (April 2011). "Xylazine (veterinary sedative) use in Puerto Rico". Substance Abuse Treatment, Prevention, and Policy. 6: 7. doi:10.1186/1747-597x-6-7. PMC 3080818. PMID 21481268.
- ^ Melia M (5 August 2010). "ACLU probes prisoner deaths in Puerto Rico". Boston.com. Associated Press. Retrieved 24 August 2016.
- ^ "Opioid Epidemic Updates: "Frankenstein Opioids" and Xylazine-Induced Skin Ulcers". aafp.org. Retrieved 23 February 2023.
- ^ "Research Topics - Xylazine". National Institute on Drug Abuse. U.S. Department of Health and Human Services. 21 April 2022. Retrieved 25 January 2023.
- ^ Russell E, Sisco E, Thomson A, Lopes J, Rybak M, Burnett M, et al. (April 2023). "Rapid Analysis of Drugs: A Pilot Surveillance System To Detect Changes in the Illicit Drug Supply To Guide Timely Harm Reduction Responses - Eight Syringe Services Programs, Maryland, November 2021-August 2022". MMWR. Morbidity and Mortality Weekly Report. 72 (17): 458–462. doi:10.15585/mmwr.mm7217a2. PMID 37104171. S2CID 258354168.
- ^ Friedman J, Montero F, Bourgois P, Wahbi R, Dye D, Goodman-Meza D, et al. (April 2022). "Xylazine spreads across the US: A growing component of the increasingly synthetic and polysubstance overdose crisis". Drug and Alcohol Dependence. 233: 109380. doi:10.1016/j.drugalcdep.2022.109380. PMC 9128597. PMID 35247724.
- ^ Ehrman-Dupre R, Kaigh C, Salzman M, Haroz R, Peterson LK, Schmidt R (2022). "Management of Xylazine Withdrawal in a Hospitalized Patient: A Case Report". Journal of Addiction Medicine. 16 (5): 595–598. doi:10.1097/ADM.0000000000000955. PMID 35020700. S2CID 245907730.
- ^ Weixel N (2023-04-12). "White House says fentanyl laced with 'tranq' drug is 'emerging threat'". The Hill. Retrieved 2023-04-13.
- ^ Rock KL, Lawson AJ, Duffy J, Mellor A, Treble R, Copeland CS (July 2023). "The first drug-related death associated with xylazine use in the UK and Europe". Journal of Forensic and Legal Medicine. 97: 102542. doi:10.1016/j.jflm.2023.102542. ISSN 1752-928X. PMID 37236142. S2CID 258859939.
- ^ "Xylazine: Flesh-rotting 'zombie' drug claims first victim in Europe". euronews. May 26, 2023.
- ^ Copeland CS, Rice K, Rock KL, Hudson S, Streete P, Lawson AJ, et al. (April 2024). "Broad evidence of xylazine in the UK illicit drug market beyond heroin supplies: Triangulating from toxicology, drug-testing and law enforcement". Addiction. doi:10.1111/add.16466.
- ^ "'Zombie' drug xylazine found in cannabis THC vapes in UK". BBC News. Apr 10, 2024.
- ^ Ball NS, Knable BM, Relich TA, Smathers AN, Gionfriddo M, Nemecek BD, et al. (August 2022). "Xylazine poisoning: a systematic review". Clinical Toxicology. 60 (8): 892–901. doi:10.1080/15563650.2022.2063135. PMID 35442125. S2CID 248264717.
- ^ Hoffman J (7 January 2023). "Tranq Dope: Animal Sedative Mixed With Fentanyl Brings Fresh Horror to U.S. Drug Zones". The New York Times. Retrieved 23 February 2023.
- ^ "Xylazine Public Health Advisory, November 2022" (PDF). Boston Public Health Commission. City of Boston. Retrieved 25 January 2023.
- ^ Scheinin H, Aantaa R, Anttila M, Hakola P, Helminen A, Karhuvaara S (September 1998). "Reversal of the sedative and sympatholytic effects of dexmedetomidine with a specific alpha2-adrenoceptor antagonist atipamezole: a pharmacodynamic and kinetic study in healthy volunteers". Anesthesiology. 89 (3): 574–584. doi:10.1097/00000542-199809000-00005. PMID 9743392. S2CID 25346350.
- ^ Pertovaara A, Haapalinna A, Sirviö J, Virtanen R (2005). "Pharmacological properties, central nervous system effects, and potential therapeutic applications of atipamezole, a selective alpha2-adrenoceptor antagonist". CNS Drug Reviews. 11 (3): 273–288. doi:10.1111/j.1527-3458.2005.tb00047.x. PMC 6741735. PMID 16389294.
- ^ Ndeereh DR, Mbithi PM, Kihurani DO (June 2001). "The reversal of xylazine hydrochloride by yohimbine and 4-aminopyridine in goats". Journal of the South African Veterinary Association. 72 (2): 64–67. doi:10.4102/jsava.v72i2.618. PMID 11513261.
- ^ "FDA alerts health care professionals of risks to patients exposed to xylazine in illicit drugs". Center for Drug Evaluation and Research. U.S. Food and Drug Administration. 2022-11-08.
Further reading
[edit]- McCurnin DM, Bassert JM (2002). Clinical Textbook for Veterinary Technicians (5th ed.). Philadelphia: Saunders.
- "Rompun Homepage". Bayer Healthcare. 2005. Archived from the original on 2007-03-02.
- Wright B (2000). "Human health concerns when working with medications around horses". Agdex# 460. Ontario Ministry of Agriculture, Food and Rural Affairs (OMAFRA). Archived from the original on 7 September 2006.
